Method for avoiding corrosion of dissimilar metals is to put the contact surfaces of the dissimilar metals in a dry location so electrolytes will not be present in the contact areas. This dissimilar-metal couple can produce macro-scopic galvanic corrosion.

Synergy Of Erosion And Galvanic Effects Of Dissimilar Steel Welding Field Failure Analysis Case Study And Laboratory Test Results Sciencedirect
When two different metals are in contact and exposed to moisture containing salts or pollutants one of the metals can experience accelerated corrosion while the other remains protected.
Welding dissimilar metals galvanic corrosion. Stainless steel and galvanized materials often are found together in the industry with applications such as galvanized fasteners stainless steel pressure vessels and roof and siding panels. Dissimilar Metals and Galvanic Corrosion. An electrolyte could be any non-metal matter that will conduct an electric current and are.
The presence of two dissimilar metals in an assembly is not always a sign of trouble but it could be a problem. For example rain water and salt water make especially good electrolytes. The cell produced can result in corrosion to one of the paired metals.
The less noble of the metals functions as an anode and will corrode faster. The contact may be direct or by an external pipe or wire or bolt. Galvanic Corrosion of Dissimilar Metals.
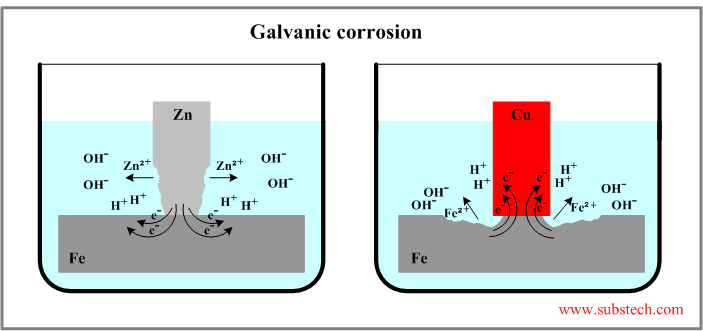
Galvanic corrosion some times called dissimilar metal corrosion is the process by which the materials in contact with each other oxidizes or corrodes. One metal the cathode is protected whilst the other the anode is corroded. The fusion zone also has a thin region adjacent to the fusion line known as the unmixed chilled.
Bimetallic corrosion can only occur when two dissimilar metals are in electrical contact and are bridged by an electrically conductive liquid. 1 Weight and material distribution in car body by may initiate galvanic corrosion in aggressive environments. Galvanic corrosion occurs when two dissimilar metals are immersed in a conductive solution and are electrically connected.
Without splitting hairs well discuss the actual problem why it is important to well owners and how a quality water system can be built to avoid it. First there must be two electrochemically dissimilar metals present. When two metals are in direct contact.
If the dissimilar metals are insulated from each other by suitable plastic strips washers or sleeves then galvanic corrosion. SLC project7 The formation of thick intermetallic compounds IMCs layer in dissimilar metal welds can increase the possi- plications11. Galvanic Corrosion is an electrochemical process which occurs when dissimilar metals are in contact with each other in the presence of an electrolyte most commonly being moisture and oxygen.
Sion process in the vicinity of the weld. This type of accelerated corrosion between dissimilar metals negatively impacts the overall corrosion protection of the project and is referred to as galvanic corrosion bi-metallic corrosion or dissimilar. Corrosion in metals deteriorates the metal parts due to chemical reactions between metal and the surrounding environment.
Galvanic corrosion is a common form of corrosion that is induced when two dissimilar metals are in direct contact with one another under water. Although problems involving dissimilar welding are rare they do exist and some of them are documented ranging from residual stress problems Joseph et al 2005 to corrosions Pimenta and Jarman 2010. More information about the use of dissimilar metals is available in the commentary of AWS D16 Structural Welding.
Galvanic Corrosion is also known as Dissimilar Metal or Bimetallic Corrosion is a type of electrochemical corrosion where a material corrodes if it comes in contact with another material in the presence of an electrolyte. This can be an issue when stainless steels are in contact with other metals depending on the circumstances. The fusion zone itself offers a microscopic galvanic effect due to microstructural segrega-tion resulting from solidification Ref 3.
To avoid undue stressing the weldmetalshouldifpossible have an expansion coefficient intermediate between the two parent alloys ie providing a buttering layer Figure 4. There are three conditions that must exist for galvanic corrosion to occur. Metal-to-metal contact Galvanic corrosion can only occur if the dissimilar metals are in electrical contact.
When a galvanic couple of this type is formed the more positive noble metal functions as a cathode and will corrode slower than it would by itself. Welding dissimilar metals has always been considered as a problem due to the different ways in which metals respond to various heats stresses strains and environments. The rate of attack on the anode is accelerated compared to the rate when the metal is uncoupled.
Fiberglass themoplastic Moreover dissimilar metal welds form galvanic couples and Fig. It is for this reason that the operating stress on dissimilar metal weld joints between stainless and carbon steels should be kept at a minimum. Corrosion on dissimilar metals is often called electrolysis but it is technically not.
Galvanic Action Corrosion Prevention Architect S Blog
21 Lovely Galvanic Corrosion Chart Dissimilar Metals Chart Gallery

Galvanic Series Electrochemical Series

Right Combination Of Metals Steels And Alloys To Avoid Bimetallic Corrosion Galvanic Corrosion Food Protection And Security Preventing And Mitigating Contamination During Food Processing And Pro

Corrosion Behaviors Of Friction Welded Dissimilar Aluminum Alloys Sciencedirect

Faq 1 Galvanic Dissimilar Metal Corrosion

Synergy Of Erosion And Galvanic Effects Of Dissimilar Steel Welding Field Failure Analysis Case Study And Laboratory Test Results Sciencedirect
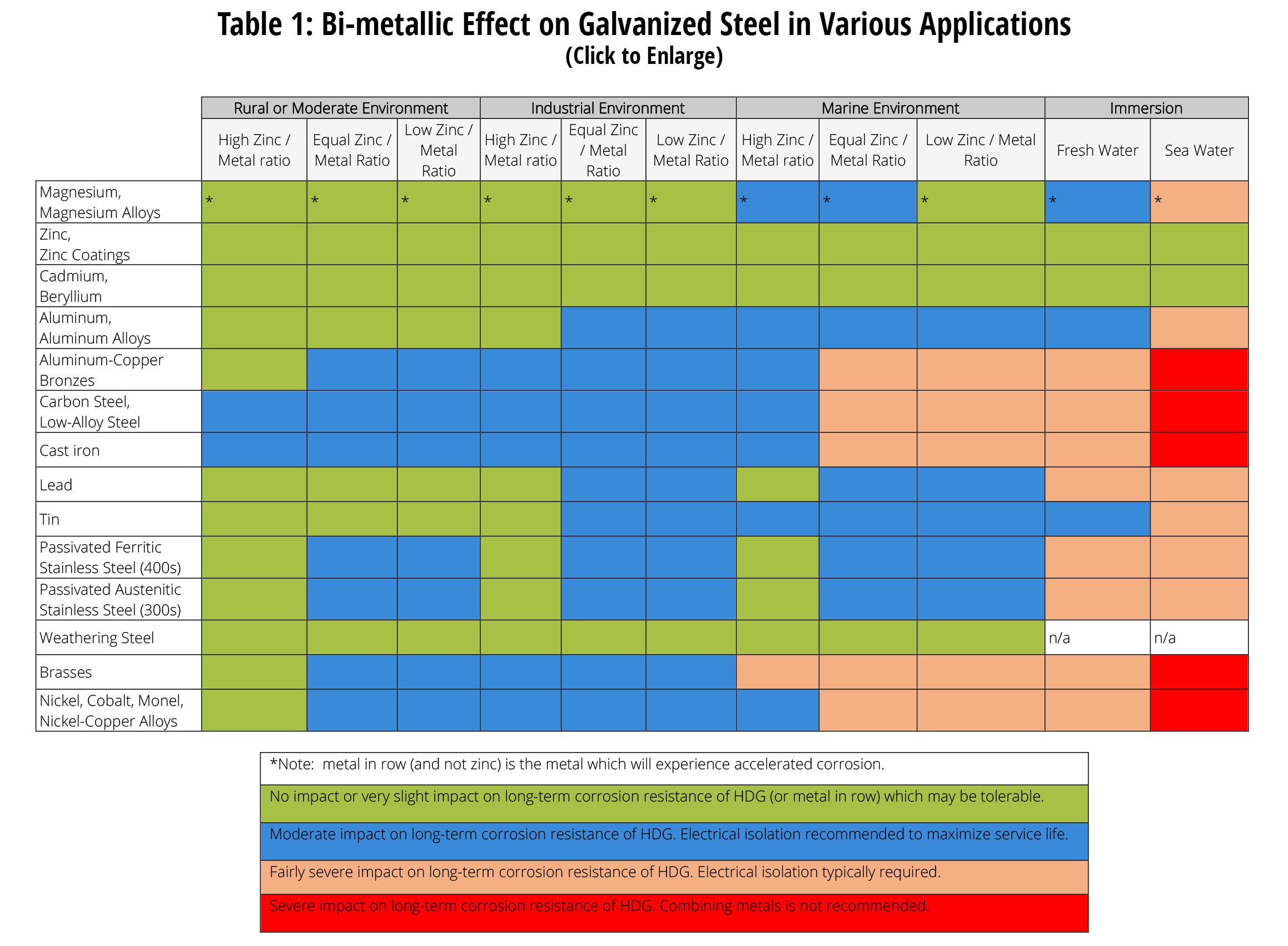
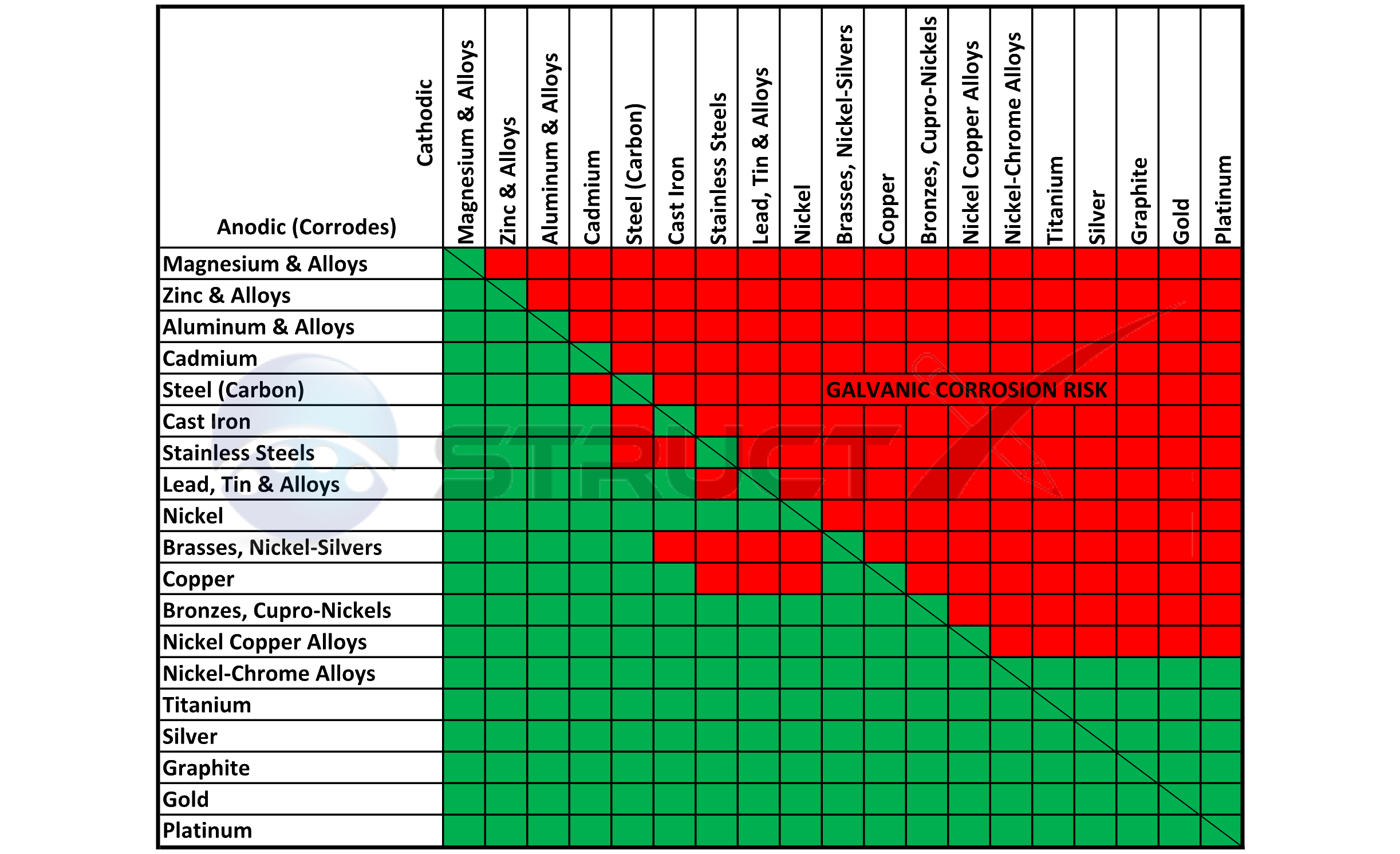
Dissimilar Metals In Contact With American Galvanizers Association
0 comments:
Post a Comment